A 67-year-old female presents with worsening shortness of breath, fever, and hypoxemia requiring mechanical ventilation. She was transferred from a Long-Term Acute Care Facility with a chronic tracheostomy.
She recently completed a course of meropenem for a urinary tract infection. Chest X-ray shows an infiltrate in the left lower lobe.
Ceftazidime/Avibactam was started, but 24 hours later, her oxygenation continued to worsen, and purulent secretions were noted.
A bronchoscopy was performed. The organism below was identified from bronchoscopy specimens:
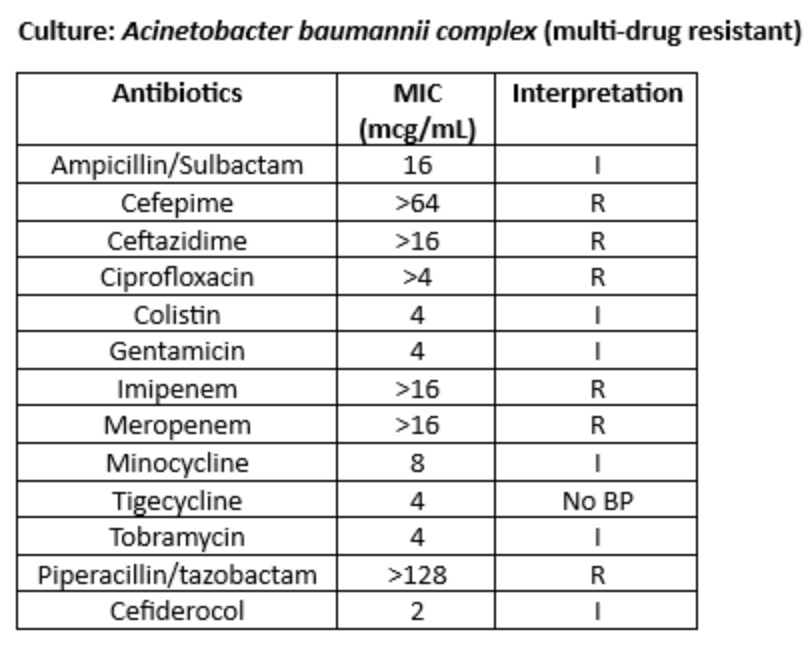
Ceftazidime/Avibactam was changed to sulbactam/durlobactam, and meropenem was added for synergy.
The initial management step is to determine if a culture with resistance to multiple antimicrobials requires treatment at all. Cultures are frequently taken from respiratory secretions or wounds and can represent colonization instead of true infection.
Management of infections due to carbapenem-resistant Acinetobacter baumannii (CRAB) requires aggressive management. This is largely due to common, intrinsic resistance mechanisms making antibiotic management complicated. These intrinsic resistance mechanisms, specifically AmpC and various OXA enzymes, may develop while patients are on therapy. This frequently results in the utilization of dual or triple therapy when a CRAB is a multi-drug resistant (MDR) or extensively drug resistant (XDR) organism.
Resistance mechanisms seen in Acinetobacter baumannii:
Beta lactams
Aminoglycosides
Quinolones
Biofilm formation is common as well, thus increasing treatment complexity beyond the resistance limitations above.
Treatment options:
CRAB infections are highly resistant to multiple classes of antibiotics, leaving few options in the arsenal for treatment.
IDSA treatment guidelines recommend using combination therapy for the treatment of CRAB. Combination therapy is needed for many reasons, but theories include synergistic antibiotic treatment, preventing intrinsic resistance leading to inducible MIC creep, and overcoming heteroresistance within numerous antibiotic classes. The lack of comparator studies does not point the clinician to a specific preferred regimen. The guidelines note that only one trial (of eight studies reviewed) demonstrated improved clinical outcomes with antimicrobials in combination [1].
Ampicillin-Sulbactam (High Dose)
The antibiotic is dosed based on sulbactam component at 9 grams every 8 hours with extended infusion or 27 grams continuously. This is currently the suggested approach by IDSA [1]. High-dose ampicillin-sulbactam is used to saturate the PBP targets of sulbactam, thereby overcoming resistance commonly seen on sensitivity panels. A review article indicated that high dose sulbactam with another agent(s) was a reasonable option for CRAB [2]. The authors noted that additional high-quality studies were needed to confirm clinical efficacy.
Polymyxins/Colistin
Polymyxins, commonly colistin, are used to treat CRAB. In one trial comparing colistin (9 million unit loading dose, followed by 4.5 million units twice per day to colistin with meropenem (2g prolonged infusion three times per day), the clinical failure rates were 83% in the colistin monotherapy arm. The secondary outcome of 28-day mortality was 46% [3]. This data suggests that colistin monotherapy has no role. Polymyxins may be considered, but dosing must be higher than conventional strategies to overcome any serum level variability. [4] Polymyxins have low penetration into lung tissue and low serum concentrations, which make them a poor monotherapy agent for lower respiratory tract infections.
Tetracyclines and derivatives
(Minocycline/Tigecycline/Omadacycline/Eravacycline)
Many tetracycline derivatives have potent activity against MDR CRAB. The cornerstone of treatment was minocycline for many years. Minocycline has shown synergistic activity with high-dose sulbactam, polymyxins, and carbapenems. Minocycline plus high-dose sulbactam has been shown to lower MICs to the high-dose sulbactam by upwards of 1-2 fold [5]. Both Omadacyline and Eravacycline have shown potent activity against CRAB. However, it is important to note that omadacyline appears susceptible to the common tetracycline resistance mechanisms, whereas eravacycline retains activity in these situations [6,7]. Lastly, tigecycline usage is difficult since it has a low serum concentration of the drug, an unfavorable side effect profile, and poor target attainment for most MICs. [8].
Cefiderocol
Although cefiderocol has favorable in vitro activity, this drug has not fared well in clinical studies. Specifically, although clinical cure rates were similar between cefiderocol and the best available treatment groups, mortality in the Acinetobacter subgroup was higher [9]. Another trial showed non-inferiority compared to high-dose extended infusion meropenem for carbapenem-resistant organisms, but a subgroup analysis for Acinetobacter favored the meropenem arm [10]. A small real-world observational trial of 11 patients utilizing cefiderocol in combination with colistin did show clinical success in eight of the 11 patients [11]. Increased failure rates for cefiderocol may be related to heteroresistance. This may lead patients to develop spontaneous resistance during therapy, leading to potential treatment failure. Accordingly, cefiderocol is relegated to the position of a second-line agent for CRAB
Sulbactam-Durlobactam (SUL-DUR)
This is the newest agent approved by the FDA for the treatment of Acinetobacter. A combination of sulbactam and a new beta-lactamase inhibitor, durlobactam, was specifically targeted to treat CRAB. Durlobactam lowers sulbactam MICs up to 32-fold against CRAB [12]. The drug was approved on the back of the ATTACK non-inferiority trial. SUL-DUR plus imipenem/cilastatin versus colistin plus imipenem/cilastatin for CRAB was shown to be non-inferior. SUL-DUR had an improved microbiological cure rate compared to the colistin group but with higher recurrence rates at late follow-up. The number of recurrences was small, affecting six (10%) patients in the SUL-DUR group vs two (3%) patients in the colistin group. The treatment difference was not calculated, making it difficult to determine the clinical value of this finding. [13]. Of note, there have been numerous cases documenting the synergism between carbapenems and sulbactam, lowering the MIC in vitro [14–16]. It is unclear if this change is clinically meaningful or will be a necessary addition when using SUL-DUR. However, cost and formularies will likely exclude this therapy from being the standard of care until more data is published showing superiority over other current regimens.
Alternate regimens:
Triple drug therapy: high dose carbapenem, polymyxin B, sulbactam.
In a small study, this therapy was given to colistin-resistant patients, with no deaths observed across seven patients versus six of ten patients expiring in the SOC arm. [15]. Another group employed this triple drug strategy, with three of 13 patients (39%) experiencing 30-day mortality and only two of ten achieving microbiologic cure [17]. Even though the study numbers are small, this regimen may be an option for organizations with high levels of colistin-resistant organisms.
Much of the data in use to support our current regimens for the treatment of CRAB is based on in vitro data with very few head-to-head studies. Although sulbactam-based regimens are currently the preferred regimen of choice, the poor overall clinical cure and mortality rates, as well as the numerous CRAB resistance mechanisms, will require new antimicrobial options in the future.
