Abstract
Patients with COVID-19 pneumonia on mechanical ventilation can exhibit clinical signs difficult to distinguish from ventilator-associated pneumonia (VAP). Positive sputum cultures in these patients often lead to the use of broad-spectrum antibiotics. Therefore, we aim to evaluate the clinical utility and efficacy of antimicrobial coverage for positive sputum cultures in mechanically ventilated patients with COVID-19. These subjects (n = 98) were on mechanical ventilation and had positive sputum culture after 48 h of intubation during 15 March 2020–25 May 2020 at Rush University Medical Center in Chicago, IL. Only one patient did not receive antibiotics. The primary outcome was defined as the change in Sequential Organ Failure Assessment (delta SOFA score) which was calculated by comparing the SOFA score on the day of sputum culture collection with the score at 48 h and 7 days after the initiation of treatment. There were no statistically significant delta SOFA scores after 48 h of antibiotics administration. Statistically significant changes were observed after 7 days of treatment, which could be reflective of an improvement in viral pneumonia with ICU supportive care. Physicians should consider that positive sputum cultures may not always indicate VAP and apply clinical judgement to avoid the overuse of broad-spectrum antibiotics in critically ill patients with COVID-19.
Introduction
Patients with COVID-19-associated respiratory infections exhibit varying degrees of disease severity. Although most patients have mild or uncomplicated disease [1], those with severe disease have been noted to develop shock, multi-organ failure and acute respiratory distress syndrome (ARDS), raising clinical suspicion for superimposed ventilator-associated pneumonia. Despite the low rates of reported bacterial and fungal co-infections in COVID-19 patients, the widespread use of empiric broad-spectrum antimicrobials has been observed [2]. A review article by Rawson et al. reports that 72% (1450/2010) of patients with COVID-19 were treated with antimicrobials empirically despite a lack of evidence of bacterial or fungal co-infection [3]. Only 8% (62/806) of patients with COVID-19 were reported as experiencing co-infection during hospital admission. Other studies have reported secondary infection in 13.5–44% of COVID-19 patients in the intensive care unit (ICU), of which the most common type of infection was pneumonia [4]. In a report from 552 hospitals in 30 Chinese provinces, empiric antimicrobial usage was likely widespread because 25–70% of severely ill COVID-19 patients manifested evidence of sepsis, and it was difficult to exclude bacterial or fungal superinfections based on signs and symptoms, physical findings, radiographic abnormalities, and laboratory results [5]. Incidence of superinfections may have been overstated by a failure to distinguish colonization from disease or were understated by high mortality rates among ICU patients (ranging from 16–78%) and insufficient patient follow-up among survivors [4].Positive sputum cultures may frequently indicate the colonization of bacteria in the upper nasopharynx and endotracheal tube (ETT) instead of a true pathogen. Our anecdotal experience with critically ill COVID-19 patients at Rush University Medical Center in Chicago, IL., suggested that antimicrobial coverage to target organisms identified in sputum cultures did not seem to change the clinical course in many cases. With possible unintended consequences associated with antimicrobial use, there is an increased need for optimal treatment guidelines and antimicrobial stewardship for COVID-19 patients in the ICU. We designed this study to evaluate the clinical utility and efficacy of antimicrobial coverage for positive sputum cultures in ventilated COVID-19 patients to decrease the inappropriate use of antimicrobials, avoid the development of multidrug-resistant organisms (MDRO) and reduce antimicrobial side effects.
Methods
Study Design and Population
VAP is defined as pneumonia that occurs 48 h after intubation and mechanical ventilation. The diagnosis of VAP requires clinical findings of new or progressive infiltrates, worsening oxygenation and a change in the quality or quantity of sputum which is combined with microbiologic analysis of respiratory secretions. The diagnosis of VAP in patients and the initiation of antibiotics were decided by individual providers at Rush University Medical Center. We performed a retrospective cohort study by chart review. Subjects were patients who were at least 18 years of age or older and were admitted to an intensive care unit (ICU) at Rush University Medical Center from 15 March 2020 to 25 May 2020. Additional inclusion criteria were the following: SARS-CoV-2 PCR positive at Rush University Medical Center or SARS-CoV-2 PCR positive confirmed result from another institution, and a sputum culture ordered 48 h after intubation with a positive result. Patients who had positive sputum culture but only grew Candida species were excluded.
The primary outcome was defined as the change in Sequential Organ Failure Assessment (SOFA) score, also known as delta SOFA score, which was calculated by comparing the SOFA score on the day of sputum culture collection to the score at 48 h and 7 days after the initiation of treatment [6,7]. Secondary outcomes included delta SOFA score at 30 days after the start day of antibiotic treatment, ordinal scale of improvement in oxygen requirement, and an improvement in fever, shock, leukocytosis, and radiographic changes at 48 h and 7 days after initiation of treatment.
Sequential assessment of SOFA score during the first few days, especially the first 48 h of ICU admission, was found to be a good indicator of prognosis, independent of the initial score [7]. Boeck et al. demonstrated that outcome in VAP is accurately predicted by serial SOFA scores [8]. Therefore, we designed the study to follow serial SOFA score at 48 h, 7 days and 30 days to follow clinical course and response to antimicrobial therapy in our study population.
We initially intended to select a control group of patients who met all inclusion criteria but did not receive antibiotic treatment; however, there were not enough patients to establish a control group. Then, attempts were made to select a comparison group with patients who were COVID-19 positive with negative sputum cultures and received antibiotics, but there were insufficient numbers of patients to make the comparison. The study period was during the first wave of the pandemic when most hospitalized patients at our institution were COVID-19 patients. Therefore, we designed the study for
analysis within the same group.
Data Collection
Sputum culture data for 15 March 2020 to 25 May 2020 were obtained from the microbiology laboratory. Patients who had positive sputum culture with bacterial growth were selected and reviewed for inclusion and exclusion criteria. A total of 98 patients were screened to be eligible. Only one patient did not receive antibiotics, who was excluded from the study.
The electronic medical record system was reviewed to collect data for all study patients. The collected data were a result of routine care. Baseline characteristics (age, gender, BMI, race, ethnicity and past medical history), microorganisms identified on sputum cultures, blood cultures, the duration of antimicrobial treatment, COVID-19 pneumonia treatment, ICU and hospital length of stay, and mortality data were collected. If there were multiple sputum cultures, the first sputum culture that met the definition of ventilator-associated pneumonia (VAP) was selected and the subsequent culture results were also recorded. Procalcitonin levels, whenever available, were recorded as well. The empiric antibiotic treatment used for treatment of VAP, the duration of treatment and the duration of appropriate antibiotic therapy were collected. All patients were given empiric broad spectrum antibiotics for VAP (i.e., vancomycin and piperacillin tazobactam, or vancomycin with cefepime), before de-escalation to a targeted therapy.
The following parameters to assess clinical improvement were collected as well: parameters to yield SOFA score, respiratory status, fever, shock, leukocytosis, and radiographic changes on the day of sputum collection, 48 h, 7 days and 30 days after the initiation of treatment. SOFA scores were calculated and the changes in SOFA score (delta SOFA score) were also used to analyze the associated characteristics of patients based on delta SOFA score. The ordinal scale of overall clinical status (1–6) was applied based on oxygenation status, death or discharge. Changes in fever, shock, leukocytosis, and radiographic findings were also scored 1–4 depending on the improvement. Radiographic changes were assessed based on official readings from radiologists at our institution.
Data Analysis
The primary and secondary outcomes were obtained with baseline characteristic covariable adjustment. We also obtained the imputed data to minimize errors by implying a SOFA score of 0 if the patient was discharged at the corresponding time, and a maximum SOFA score (24) if the patient had expired.
Patients who received treatment for COVID-19 were classified based on the treatment, and the outcomes of delta SOFA score with antibiotic administration were compared between the four groups (Table S1).
We further analyzed the characteristics of patients who had bigger changes in SOFA score (higher delta SOFA score) and compared them with the characteristics of patients who had smaller changes in SOFA score (lower delta SOFA score). We compared the patients in the higher 50th percentile with the patients in the lower 50th percentile by using a median delta SOFA score of (−1) at 7 days after antibiotic therapy.
Statistical Analysis
Categorical data were presented as percentage frequencies, with continuous data presented as mean (standard deviation, sd). Categorical outcomes were analyzed using a Chi square test. Continuous outcomes were analyzed by the Mann–Whitney U test. Changes from baseline were tested by using Wilcoxon signed-rank test. Two-tailed tests were used. Statistical significance was defined as p < 0.05. Analyses were performed with SAS v9.4 (SAS, Cary, NC, USA).
Results
Characteristics of the Study Population
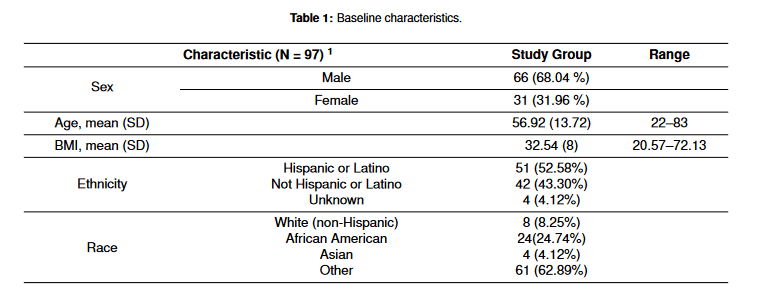
The baseline characteristics of the study population (n = 97) are shown in Table 1. The mean age was 56.92 ± 13.72 and 66 (68.04%) were male. The mean BMI was 32.54 ± 8.51. A total of 51 (52.58%) patients were Hispanic and 24 (24.74%) patients were African American. There were four (4.12%) immunocompromised patients, and all had history of transplant with one liver transplant and three renal transplants. Sputum culture grew Staphylococcus aureus in 37 (38.14%) patients and Pseudomonas aeruginosa in 13 (13.40%) patients. Sputum cultures were repeated in 62 patients and 35 (56.45%) patients grew the same organism found in the initial sputum culture despite the adequate antimicrobial coverage. A total of 12 (12.37%) patients developed bacteremia with the same organism that grew in sputum. Mean hospital days were 31.14 ± 15.23. The mean baseline SOFA score was 11.58. The mortality rate was 41.30%. Nine patients were on extracorporeal membrane oxygenation (ECMO). There were three occurrences (3%) where bacterial resistance developed during the time of antibiotic treatment. No Clostridioides difficile cases were observed in 30 days.


Primary Outcome
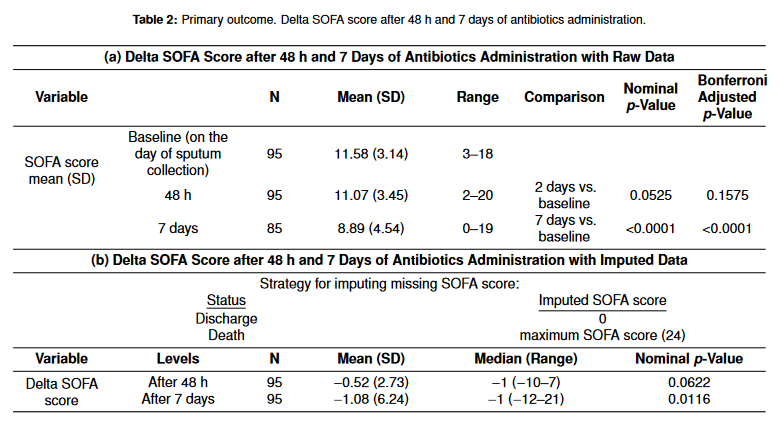
Primary outcomes are shown in Table 2. There were no statistically significant changes in SOFA score after 48 h of antibiotic administration. SOFA score after 7 days of antibiotic administration was 8.89 and this was a statistically significant change from the baseline SOFA score (Table 2a). To decrease errors from the missing SOFA score due to death or discharge, we obtained imputed data by applying a SOFA score of 0 to the discharged patients and a maximum SOFA score of 24 for patients who expired after 48 h or 7 days of antibiotic administration (Table 2b). The delta SOFA score after 48 h of antibiotic administration was not statistically significant (p = 0.0622) even after imputing missing SOFA scores.
Secondary Outcome
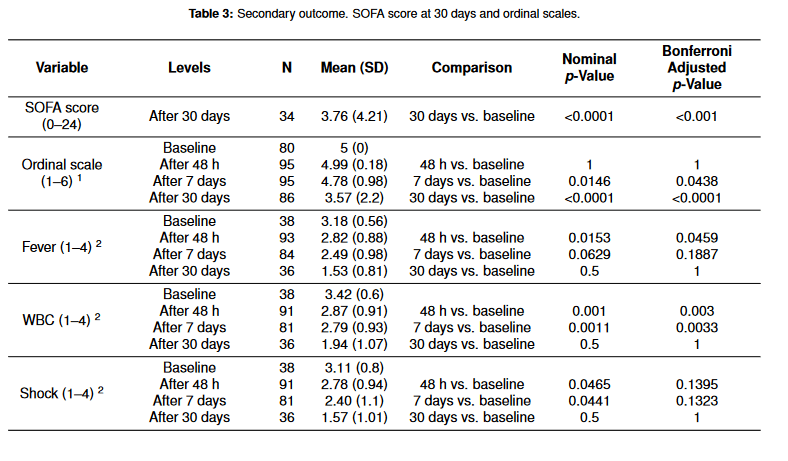
Secondary outcomes are demonstrated in Table 3. The mean SOFA score 30 days after antibiotic administration was 3.76. The overall clinical status and changes in fever, shock, leukocytosis, and radiographic findings are demonstrated in the scoring system as described under the table. Most clinical parameters (fever, WBC, shock, and respiration) were improved at 48 h and 7 days after treatment.

Patients who received treatment for COVID-19 therapy were classified based on the treatment, and the outcomes of delta SOFA score with antibiotic administration are compared between the four groups (Table S1). The study period was early in the pandemic prior to the establishment of current standard therapy (i.e., corticosteroids [9], remdesivir, tocilizumab, etc.), but rather when steroid therapy was avoided. Steroids were only used in four patients, with other therapeutic agents, and thus are not grouped separately.
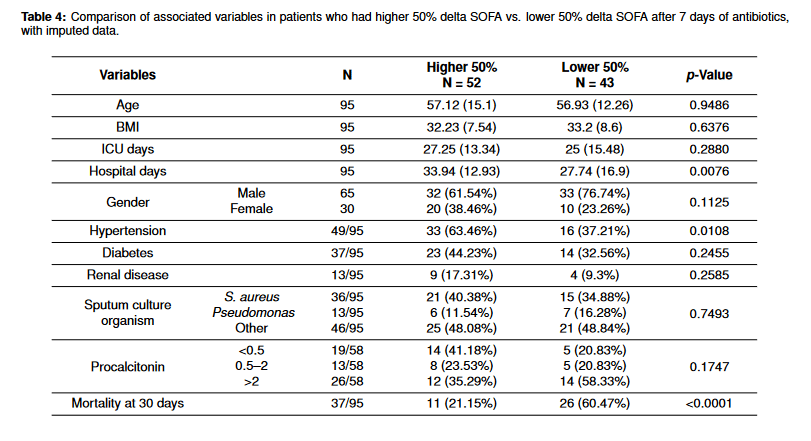
Lastly, we performed an additional analysis to compare characteristics in patients with delta SOFA score in the higher 50th percentile and the lower 50 percentile to see if there were any associated factors in treatment response and clinical improvement as measured by delta SOFA score (Table 4). The patients in the higher 50% had a shorter hospital stay (12.93 vs. 16.9), and lower mortality (21.15% vs. 60.47%). Hypertension was more prevalent in the higher 50% delta SOFA group. Besides these, there were no significantly different factors between these two groups. Patients who had greater clinical improvement had a lower procalcitonin level (<0.5), though this result was not statistically significant.
Discussion
There has been extensive use of antibiotics during the COVID-19 pandemic. Though the tendency to use antibiotics empirically at the time of COVID-19 diagnosis has reduced as the understanding of viral pneumonia has improved, antibiotic use in mechanically ventilated COVID-19 patients with positive sputum culture has remained a difficult area to practice antibiotic stewardship. Patients with critical COVID-19 infection often exhibit the overlapping clinical features of ventilator associated pneumonia, and given their critically ill clinical status, broad-spectrum antibiotics have been generously used.
Although many studies described VAP in COVID-19 patients, to our knowledge, this is the first study to retrospectively evaluate clinical response to antibiotic therapy in COVID-19 patients with positive sputum culture who were treated as VAP.
Primary analysis showed there was no statistically significant improvement at 48 h after antibiotic initiation, but there was improvement after 7 days with both raw and imputed data. SOFA score changes at 48 h after antibiotics treatment would be expected in the treatment course of most bacterial pneumonias. The clinical improvement after 7 days could be related to the improvement in COVID-19 infection with ICU supportive care and thus it is difficult to attribute the improvement to the use of antibiotics alone.
Our study had several limitations. First, all of our study patients were in the ICU and received ICU supportive care, including, but not limited to, pressor therapy, antipyretics, renal replacement, and fluid administration, factors that could have affected SOFA scores. Second, we did not have a control group as nearly all patients (97 out of 98 patients) received antibiotics following the positive sputum culture. Third, we had to rely on data that had already been gathered and objectively measured, thus some of the data that are commonly used to guide diagnosis of VAP (e.g., discolored or purulent sputum, changes in sputum amount, etc.), were not available for our study.
With the performed study, we cannot conclude that empiric antibiotic usage would make a difference in the clinical improvement of critically ill COVID-19 patients with positive sputum cultures. First, there was no significant SOFA score change at 48 h after antibiotic treatment, which would be expected for most bacterial pneumonias. Clinical improvement after 7 days is likely related to the overall improvement of COVID-19 infection. Second, the group with high delta SOFA scores had a higher number of patients with low procalcitonin (<0.5), suggesting that 41.18% of patients who had greater clinical response were unlikely to have bacterial infections. Though this was not statistically significant due to low power (n = 58), this does raise the point that antibiotics were likely not a factor in the clinical improvement of patients with high delta SOFA score. The utility of procalcitonin (PCT) in diagnosis of VAP is limited, but PCT is still used to guide discontinuation of therapy in conjunction with clinical parameters. Although a standardized guideline is not available, most studies strongly recommend starting antibiotics when PCT > 0.5 ng/mL, and recommend antibiotics when PCT > 0.25 ng/mL, and discouraged antibiotic use when PCT < 0.1 ng/mL [10]. One study demonstrated that the procalcitonin cutoff value of >0.5 ng/ mL gave a sensitivity of 94.1% and specificity of 88.4% for diagnosis of lower respiratory tract bacterial infection [11].
Conclusions
Our study demonstrated that there were no statistically significant changes in SOFA scores in COVID-19 ICU patients who received antibiotics for positive sputum cultures after more than 48 h of intubation. Statistically significant changes were only observed after 7 days of treatment. As our study had the limitation of being retrospective and lacked a control group, we could not conclude whether or not empiric antibiotic use made a difference in critically ill COVID-19 patients with positive sputum cultures. However, there are several findings we observed that suggest mechanically ventilated patients with COVID-19 pneumonia improved likely due to ICU supportive care rather than antibiotic treatment for VAP. Physicians should consider that positive sputum cultures may not always indicate VAP and apply clinical judgement to avoid the overuse of broad-spectrum antibiotics in critically ill COVID-19 patients. Further study and efforts are needed to achieve antibiotic stewardship in this population of patients.
Supplementary Materials
Table S1. Improvement in SOFA score at 7 days after initiation of antibiotics in different COVID-19 treatment groups.
Funding
This research received no external funding. Departmental funding was received.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Rush University Medical Center on 16 June 2020. The ORA is 20060907.
Informed Consent Statement
Patient consent was waived as this study was conducted by retrospective chart review
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rothe, K.; Feihl, S. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: A retrospective cohort study in light of antibiotic stewardship. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 859–869. [CrossRef] [PubMed]
- Lansbury, L.; Lim, B. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.P. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71,2459–2468. [PubMed]
- Clancy, C.J.; Nguyen, M.H. COVID-19, superinfections and antimicrobial development: What can we expect? Clin. Infect. Dis. 2020, 71, 2736–2743. [CrossRef] [PubMed]
- Zhou, F.; Yu, T. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [CrossRef]
- Vincent, J.L.; Moreno, R. The SOFA (Sepsis-related Organ Failure Assessment) score to
describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [CrossRef] [PubMed] - Ferreira, F.L.; Bota, D.P. Serial Evaluation of the SOFA Score to Predict Outcome in Critically Ill Patients. JAMA 2001, 286, 1754–1758. [CrossRef] [PubMed]
- Boeck, L.; Eggimann, P. The Sequential Organ Failure Assessment score and copeptin for
predicting survival in ventilator-associated pneumonia. J. Crit. Care 2012, 27, 523.e1 523.e9. [CrossRef] [PubMed] - RECOVERY Collaborative Group; Horby, P.; Lim, W.S. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [CrossRef] [PubMed]
- Azzini, A.M.; Dorizzi, R.M. A 2020 review on the role of procalcitonin in different clinical settings: An update conducted with the tools of the Evidence Based Laboratory Medicine. Ann. Transl. Med. 2020, 8, 610. [CrossRef] [PubMed]
- Amal, A.E.; Gehan, H. The role of procalcitonin as a guide for the diagnosis, prognosis, and decision of antibiotic therapy for lower respiratory tract infections. Egypt. J. Chest Dis. Tuberc. 2013, 62, 687–695.

