Introduction
Actinomyces species are branching, gram-positive, facultative anaerobes that can be found in the oral cavity’s normal flora, as well as in the intestinal and female genitourinary tracts [1]. However, they can cause human pathology, typically forming fistulous tracts or slow-growing masses that invade adjacent structures. One hallmark feature of actinomycosis is the presence of sulfur granules, which are often observed in the affected tissues or drainage [2]. We report a rare case of genitourinary actinomycosis in a male patient who presented with an acute penile abscess and erosion of adjacent structures.
Case Presentation
A 38-year-old male with no significant past medical history presented with a 1-week history of scrotal and penile swelling. He reported sexual activity with multiple female partners over the past six months, with inconsistent condom use during oral and vaginal intercourse. He denied any history of genital trauma or traumatic sexual encounters. On review of systems, the patient reported having experienced fevers and chills and denied dysuria or penile discharge.
The patient had no significant past medical history or history of prior surgeries. He was born and raised in the Midwestern United States and reported no exposure to pets or other animals. He had no significant family history. He had not been taking any medications at home.
The patient arrived at the emergency department with a temperature of 100.3 ˝F, heart rate of 86
beats per minute, blood pressure of 109/61 mmHg, and an oxygen saturation of 97% on room air. On genitourinary examination, there was significant penile and scrotal pain, swelling, and erythema, with the swelling more pronounced on the left side of the penis. Prominent swelling was also noted at the inferior base of the penis and scrotum. The remainder of the physical examination was unremarkable. Admission lab work is notable for a mildly elevated white blood cell count at 14.5, and a urinalysis containing 6–10 red blood cells per high-power field and 21–50 white blood cells per high-power field.
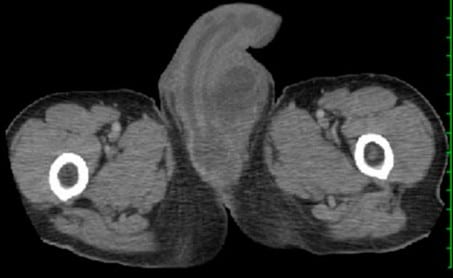
A computed tomography (CT) of the abdomen and pelvis with intravenous (IV) contrast revealed that the urinary bladder, seminal vesicles, and prostate gland were unremarkable. However, there was a large fluid collection along most of the ventral aspect of the penis. The walls of this collection showed contrast enhancement, consistent with an abscess (see Figure 1). The fluid collection extended slightly more to the left side of the penis, particularly at the suprapubic level and near the proximal end of the penile urethra. Additionally, there was diffuse soft-tissue swelling in the scrotum, with possible small bilateral hydroceles.

A testicular ultrasound revealed normal bilateral testes. However, a heterogeneously echoic collection was noted at the ventral aspect of the penile base, measuring up to 7.1 cm in diameter, without any associated vascular signal, which correlates with the CT and physical exam findings.
The patient was initially treated with vancomycin and piperacillin-tazobactam. On hospital day 3, the urology team performed a surgical debridement. Purulent drainage that was subsequently cultured was observed over the right side of the glans penis. A Foley catheter was inserted, but purulent material continued to drain. An open sinus was identified on the ventral aspect of the penis, accompanied by significant purulence. The area was debrided, revealing that the Foley catheter had been misplaced in the perineum. Further surgical examination revealed a complete breakdown of the ventral aspect of the urethra and complete obliteration of the corpus spongiosum. All purulent material was thoroughly debrided. Complications arose when the suprapubic tube failed, possibly due to dehydration. As a result, a urinary catheter was inserted into the urethra and guided to the bladder through the urethral erosion.
The patient’s laboratory workup revealed no growth on the urine culture. The rapid plasma reagin test returned positive with a titer of 1:64. The HIV antibody/antigen test was negative. Urine testing for gonorrhea was negative, while urine testing for chlamydia was positive.
One of the cultures from surgical debridement was positive for Actinomyces species and Prevotella species; a different culture was positive for Actinomyces species and Anaerococcus species.
The treatment plan included a 4-week course of IV ceftriaxone combined with oral metronidazole. Afterwards, the patient received an additional 5 months of oral amoxicillin-clavulanate. Furthermore, the patient received a 7-day course of doxycycline to treat the chlamydia infection. The genitourinary exam improved significantly at outpatient follow-up and the patient eventually required urologic reconstruction.
Discussion
Actinomycosis is a chronic bacterial infection known for its ability to spread across tissue and fascial planes, ignoring anatomical barriers, and often results in the formation of fistulous tracts, dense adhesions to adjacent structures, or slow-growing masses [2]. This behavior likely explains why the patient experienced such severe penile destruction. While the infection usually progresses slowly, with a median time of 30 days from the onset of symptoms to diagnosis, it can occasionally lead to the rapid formation of abscesses [3]. These unique clinical features contribute to the complexity and difficulty in diagnosing and managing the infection, requiring prolonged antimicrobial treatment and, in some cases, surgical intervention for the debridement of infected tissues.
The most frequently affected area is the orocervicofacial region, which accounts for 40% to 69% of cases. The next highest prevalence is in the abdominopelvic region, which includes the genitourinary tract, and accounts for 25% to 35% of cases. Infections in the thoracopulmonary region occur in about 20% to 25% of cases. Cutaneous infections are relatively rare and make up only about 3% to 5% of cases [1,4].
Genitourinary actinomycosis is a condition that is most commonly associated with intrauterine devices (IUDs) in females, especially those in place for more than 5 years. In some cases, these female patients are colonized asymptomatically and Actinomyces is found incidentally during a Pap smear [5,6]. Male genitourinary actinomycosis, on the other hand, is rare and is most likely related to penile cutaneous actinomycosis [7]. We suspect that this patient may have acquired actinomycosis via oral intercourse and subsequent cutaneous micro-breakdown of the penis given the presence of other oral anaerobic flora (ie Prevotella and Anaerococcus) on the culture of the abscess, co-infection with multiple sexually transmitted diseases, and lack of other risk factors to acquire genitourinary actinomycosis.
Treatment of actinomycosis usually depends on the severity and extent of the infection. Oral penicillin or amoxicillin is generally sufficient for mild cases of actinomyces, such as those limited to oral disease. The treatment duration for mild disease ranges from 2 to 6 months. On the other hand, severe or extensive infections require a more aggressive approach, which usually starts with IV penicillin or ceftriaxone for 4 to 6 weeks. This is usually followed by oral antibiotics, with a total treatment course completed within 6 to 12 months [8].
There are instances, however, when surgical debridement may be necessary to remove infected tissues because medical therapy as a stand-alone treatment may not be enough to achieve a full resolution of the infection [9]. It is important to note that shorter courses of antibiotics have been associated with higher risk of recurrence, highlighting the importance of adhering to the full prescribed course of treatment [8].
For patients who are allergic to penicillin, alternative antibiotic regimens include ceftriaxone, doxycycline, macrolides, or carbapenems [8]. Given the prolonged nature of treatment, patient compliance and close monitoring are crucial to ensure successful outcomes and prevent relapse. The combination of long-term antibiotic therapy and surgical intervention is a comprehensive approach that is performed together when needed. This strategy is designed to effectively manage actinomycosis and prevent the risks of recurrence.
Conclusions
This case emphasizes the high destructive potential of genitourinary actinomycosis. This infection is typically found in women with IUDs, so an infection found in males is rare and requires a high index of suspicion so as to not delay patient care. Effective management of severe actinomycosis requires a combination of prolonged antibiotic therapy and surgical debridement to address both the underlying infection and the associated tissue damage. This case highlights the importance of early multidisciplinary collaboration in the management of complex infections. Research into optimizing the length of antibiotic regimens and surgical approaches for complex cases could improve overall outcomes.
Author Contributions
Patient evaluation, L.H. and A.S.; Conceptualization, L.H. and A.S.; Writing—original draft preparation, L.H.; Writing—review and editing, A.S. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
None.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wong, V.K.; Turmezei, T.D.; Weston, V.C. Actinomycosis. BMJ 2011, 343, d6099. [CrossRef] [PubMed]
- Cintron, J.R.; Del Pino, A.; Duarte, B.; Wood, D. Abdominal actinomycosis. Dis. Colon. Rectum. 1996, 39, 105–108. [CrossRef] [PubMed]
- Bonnefond, S.; Catroux, M.; Melenotte, C.; Karkowski, L.; Rolland, L.; Trouillier, S.; Raffray, L. Clinical features of actinomycosis: A retrospective, multicenter study of 28 cases of miscellaneous presentations. Medicine 2016, 95, e3923. [CrossRef] [PubMed]
- Sharkawy, A.A.; Chow, A.W.; Brook, I. Actinomycosis: Microbiology, Epidemiology, Clinical Manifestations, and Diagnosis. Uptodate. Available online: https://www.uptodate.com/contents/ actinomycosis-microbiology-epidemiology-clinical-manifestations-and-diagnosis (accessed on 30 October 2024).
- Ferjaoui, M.A.; Arfaoui, R.; Khedhri, S.; Hannechi, M.A.; Abdessamia, K.; Samaali, K.; Fezai, W.; Salhi, M.; Malek, M.; Neji, K. Pelvic actinomycosis: A confusing diagnosis. Int. J. Surg. Case Rep. 2021, 86, 106387. [CrossRef]
- Westhoff, C. IUDs and colonization or infection with Actinomyces. Contraception 2007, 75 (6 Suppl.), S48–S50. [CrossRef]
- Gajdacs, M.; Urban, E. The Pathogenic Role of Actinomyces spp. and Related Organisms in Genitourinary Infections: Discoveries in the New, Modern Diagnostic Era. Antibiotics 2020, 9, 524. [CrossRef] [PubMed]
- Valour, F.; Sénéchal, A.; Dupieux, C.; Karsenty, J.; Lustig, S.; Breton, P.; Gleizal, A.; Boussel, L.; Laurent, F.; Braun, E.; et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect. Drug. Resist. 2014, 7, 183–197. [CrossRef]
- Song, J.U.; Park, H.Y.; Jeon, K.; Um, S.W.; Kwon, O.J.; Koh, W.J. Treatment of thoracic actinomycosis: A retrospective analysis of 40 patients. Ann. Thorac. Med. 2010, 5, 80–85. [CrossRef] [PubMed]

