Introduction
Multidrug-resistant tuberculosis (MDR TB) and extensively drug-resistant tuberculosis (XDR TB) represent a critical global health challenge. MDR TB is characterized by resistance to isoniazid and rifampin, two key first-line anti-TB drugs [1]. XDR TB is characterized by its resistance to isoniazid, rifampin, any fluoroquinolone, and at least one injectable drug, rendering it impervious to most first- and second-line treatment options [1]. Below, we present a case of a patient with a history of rheumatoid arthritis (RA) who was diagnosed with pulmonary TB and was found to have MDR TB.
Case
A 37-year-old male with a medical history of RA presented to our infectious disease clinic after testing positive for Mycobacterium tuberculosis via DNA probe on bronchial washing during bronchoscopy. Originally from the Ural Mountains in Russia, he had moved to the US approximately 12 years prior but maintained frequent travel to Russia and Ukraine. After moving to the US, he underwent a workup to start on a biologic for his RA. Notably, he had a positive QuantiFERON test before starting this treatment but was not referred to an infectious disease physician at that time as his treating physician mistakenly thought that the patient’s BCG vaccination as a child could result in a positive QuantiFERON gold. Despite the positive test, he was started on certolizumab, a biologic agent, for treatment of his RA.
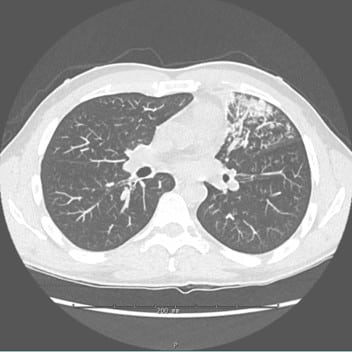
Approximately two months after starting certolizumab, he started having symptoms resembling recurrent episodes of pneumonia, with weakness, a persistent productive cough, fevers, and night sweats. Despite multiple courses of oral antibiotics prescribed for community-acquired pneumonia, his symptoms did not improve. A subsequent computed tomography scan of the chest revealed a dense consolidation in the left upper lobe, which persisted and showed possible involvement of the left lower lobe in follow-up scans (Figure 1). The patient eventually underwent bronchoscopy with bronchoalveolar lavage, confirming the presence of M. tuberculosis that took 3 weeks to grow and was positive by DNA probe. He was initially started on a 4–drug regimen consisting of isoniazid, rifapentine, moxifloxacin, and pyrazinamide (HPMZ), due to desire for a shorter overall course; however,10 days after initiation of treatment, the patient developed jaundice from Rifapentine, prompting a change to Rifampin. However, resistance testing returned 6 weeks later from the Illinois Department of Public Health and confirmed by the Centers for Disease Control and Prevention showed that the patient’s TB was resistant to isoniazid, rifampin, and ethambutol. As a result, 8 weeks after his original diagnosis, the patient’s treatment was adjusted to bedaquiline, pretomanid, and linezolid (BPaL) for a planned 26-week course.

Discussion
MDR and XDR Tuberculosis Background
The resistance mechanisms in MDR/XDR TB primarily arise from spontaneous mutations within the genome of M. tuberculosis [2]. Resistance develops through exposure to drugs, particularly in situations where treatment is administered but not monitored [2,3]. This alarming resistance trend is compounded when individuals with no prior exposure to TB drugs, as seen in our patient, encounter XDR or MDR TB patients and are infected via proximity [2]. This is particularly an issue in countries with poor infrastructure and a high prevalence of urban areas, such as low- or middle-income countries. These areas have little access to drug resistance testing, limited availability of MDR/XDR TB treatment regimens, and limited or inconsistent ability to undertake directly observed therapy [4]. Compounding the problem, many of these areas already have a high prevalence of MDR/XDR, most notably China and India, which together bear nearly 50% of the global burden of MDR/XDR cases [2]. At present, Russia, our patient’s native country, has a 14% prevalence rate of XDR TB among TB patients [3]. The combination of high prevalence, limited resources, frequent population movement, and high HIV co-infection rates makes managing and controlling MDR/XDR TB particularly challenging in such regions [4].
Treatment of XDR TB has historically proven to be an arduous task, with cure rates ranging from 40% to 50% [5]. In high-HIV-prevalent areas, these rates drop further, accompanied by higher death rates, particularly among HIV-infected individuals [6]. This issue is further complicated by the challenges of managing drug interactions between HIV treatments and TB medications, underscoring the complex interplay between MDR/XDR TB and HIV. This is specifically seen in rifamycin’s interactions with antiretrovirals spanning numerous classes, including non-nucleoside reverse transcriptase inhibitors as well as reducing the concentration of protease inhibitors [7]. Moreover, the treatment regimen spans 18–24 months and involves the use of second-line TB medications, including drugs such as clofazimine, cycloserine, or delamanid, which, though less potent and harder to tolerate [5], are essential for combating resistant strains. Even with treatment, there are still alarmingly high mortality rates, reaching 42% in some cases [6]. Other second-line treatment options have included the role of injectable drugs such as amikacin, capreomycin, and kanamycin; however, resistance has been reported to some of these choices as well [8].
Recent developments have reshaped the landscape of MDR/XDR TB treatment. Previously, guidelines recommended prolonged courses with five or more drug regimens , supported by minimal evidence that is now outdated [9]. However, emerging studies have prompted a significant shift, with new treatment protocols featuring shorter regimens and fewer drugs, signifying a positive evolution in addressing this global health crisis.
New Developments in MDR/XDR Tuberculosis Treatment
There have been many new and exciting advancements in MDR and XDR TB, one of the most interesting being the Nix trial, a randomized control trial that investigated treatment options for MDR and XDR TB using BPaL. Compared to standard TB therapies for a total duration of 26 weeks, this trial’s results showed favorable outcomes for 89% of XDR TB patients and 92% of MDR TB patients, highlighting the potential efficacy of this streamlined treatment approach [1].
However, it is important to consider the limitations of the Nix trial. Conducted exclusively in South Africa, the findings may not be universally applicable to other regions with higher prevalence, such as China, India, and Russia. Additionally, the study reported that all patients experienced adverse reactions to linezolid, highlighting the presence of challenges and drawbacks in these emerging treatment options [1].
Following the Nix trial, the BPaL regimen has been used in several studies for MDR and XDR TB [10]. These studies have influenced the WHO guidelines for treatment, which now recommends regimens with a backbone of BPaL with the addition of moxifloxacin if the patient has TB susceptible to these agents [11]. Reviews of these studies have indicated that the main reason for the discontinuation of treatment is the side effects, mostly from linezolid. However, there appear to be fewer side effects when starting at lower doses of linezolid [10].
In summary, the landscape of MDR and XDR TB treatment is evolving, with shorter and more manageable regimens offering hope for improved patient outcomes. Nevertheless, it is essential to recognize that these advancements come with their own sets of limitations and potential side effects, emphasizing the ongoing need for research and refinement in the field of TB treatment.
Author Contributions
Conceptualization, M.G., and E.A.; writing—original draft preparation, M.G., and E.A.; writing—review and editing, M.G., E.A., and G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Conradie, F.; Diacon, A.H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A.M.; Mendel, C.M.; Egizi, E.; Moreira, J.; Timm, J.; et al. Treatment of highly drug-resistant pulmonary tuberculosis. N. Engl. J. Med. 2020, 382, 893–902. [CrossRef]
- Seung, K.J.; Keshavjee, S.; Rich, M.L. Multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis. Cold Spring Harb. Perspect. Med. 2015, 5, a017863. [CrossRef] [PubMed]
- Hoffman, M. Drug-Resistant TB in Russia. Available online: https://www.wilsoncenter.org/event/ drug-resistant-tb-russia#:~:text=Russia%20has%20a%2014%20percent,highest%20rates% 20in%20the%20world (accessed on 25 May 2024).
- Ballif, M.; Nhandu, V.; Wood, R.; Dusingize, J.C.; Carter, E.J.; Cortes, C.P.; McGowan, C.C.; Diero, L.; Graber, C.; Renner, L.; et al. Detection and management of drug-resistant tuberculosis in HIV-infected patients from lower income countries. Int. J. Tuberc. Lung Dis. 2014, 18, 1327–1336. [CrossRef]
- Extensively Drug Resistant Tuberculosis (XDR TB) Fact Sheet. Available online: https://www.cdc.gov/tb/publications/factsheets/drtb/xdrtb.html (accessed on 29 January 2024).
- Gandhi, N.R.; Andrews, J.R.; Brust, J.C.; Montreuil, R.; Weissman, D.; Heo, M.; Moll, A.P.; Friedland, G.H.; et al. Risk factors for mortality among MDR- and XDR-TB patients in a high HIV-prevalence setting. Int. J. Tuberc. Lung Dis. 2012, 16, 90–97. [CrossRef] [PubMed]
- U. S. Justesen; Andersen, å.B.; Klitgaard, N.A.; Brøsen, K.; Gerstoft, J.; Pedersen, C. Pharmacokinetic interaction between rifampin and the combination of indinavir and low-dose ritonavir in HIV-infected patients. Clin. Infect. Dis. 2004, 38, 426–429. [CrossRef] [PubMed]
- Mushtaq, F.; Raza, S.M.; Ahmad, A.; Aslam, H.; Adeel, A.; Saleem, S.; Ahmad, I. Antimicrobial drug resistant features of Mycobacterium tuberculosis associated with treatment failure. PLoS ONE 2023, 18, e0293194. [CrossRef] [PubMed]
- Treatment strategies for MDR-TB and XDR-TB. In Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis; World Health Organization: Geneva, Switzerland, 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK247431/.
- WHO Operational Handbook on Tuberculosis. Module 4: Treatment—Drug Resistant Tuberculosis Treatment. Available online: https://www.who.int/publications/i/item/9789240065116 (accessed on 4 April 2024).
- Hasan, T.; Medcalf, E.; Nyang’wa, B.T.; Egizi, E.; Berry, C.; Dodd, M.; Foraida, S.; Gegia, M.; Li, M.; Mirzayev, F.; et al. The safety and tolerability of linezolid in novel short-course regimens containing bedaquiline, pretomanid, and linezolid to treat rifampicin-resistant tuberculosis: an individual patient data meta-analysis. Clin. Infect. Dis. 2024, 78, 730–741. [CrossRef] [PubMed]

